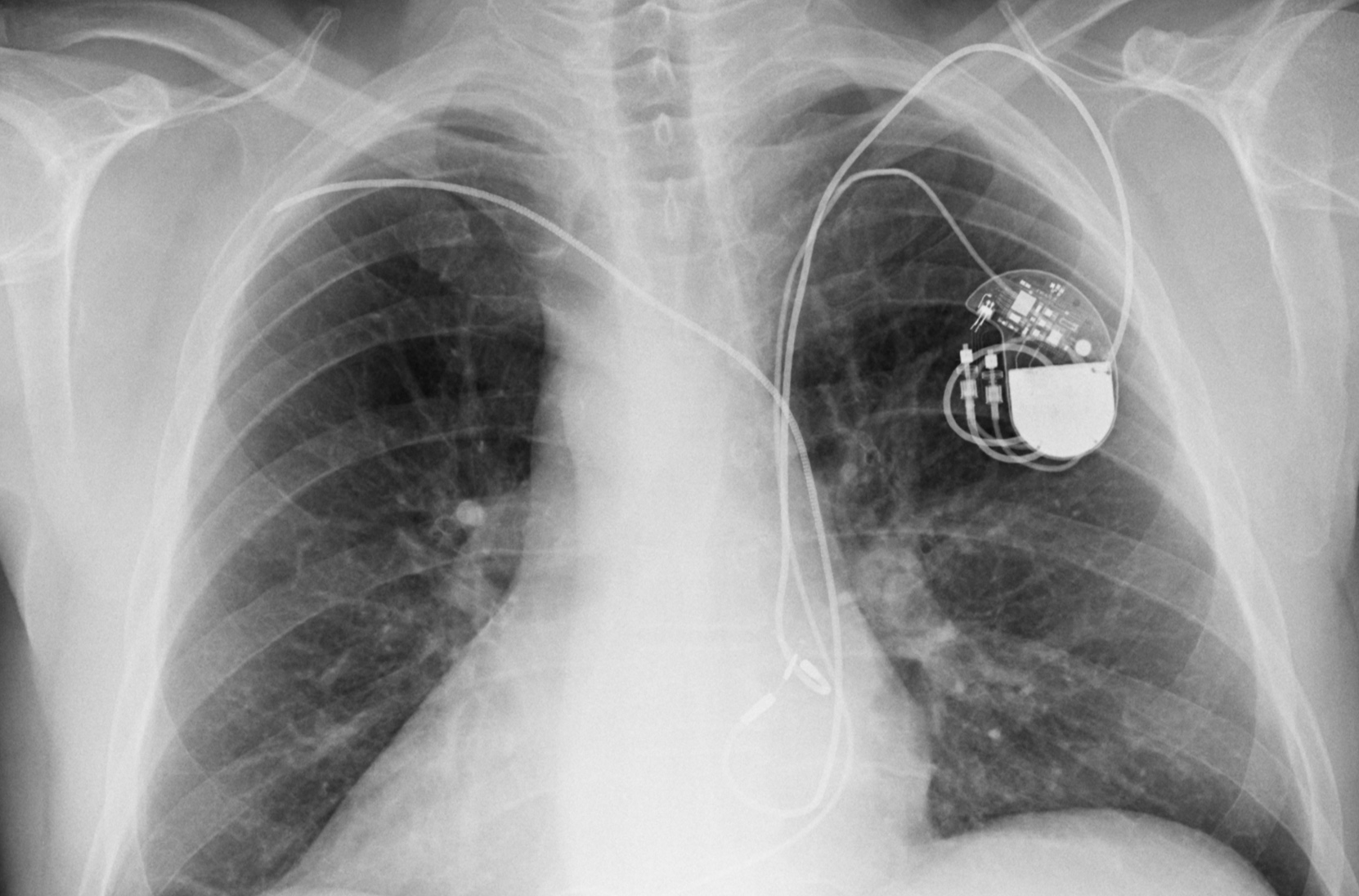
Perioperative Interrogation of Medtronic Cardiovascular Implantable Electronic Devices: A Guide for Anesthesiologists

Perioperative Interrogation of Medtronic Cardiovascular Implantable Electronic Devices: A Guide for Anesthesiologists - ScienceDirect

Appropriate Implantable Cardioverter-Defibrillator Therapies Delivered 5 Years After End of Service | JACC: Case Reports

Predicted longevity of contemporary cardiac implantable electronic devices: A call for industry-wide “standardized” reporting - ScienceDirect

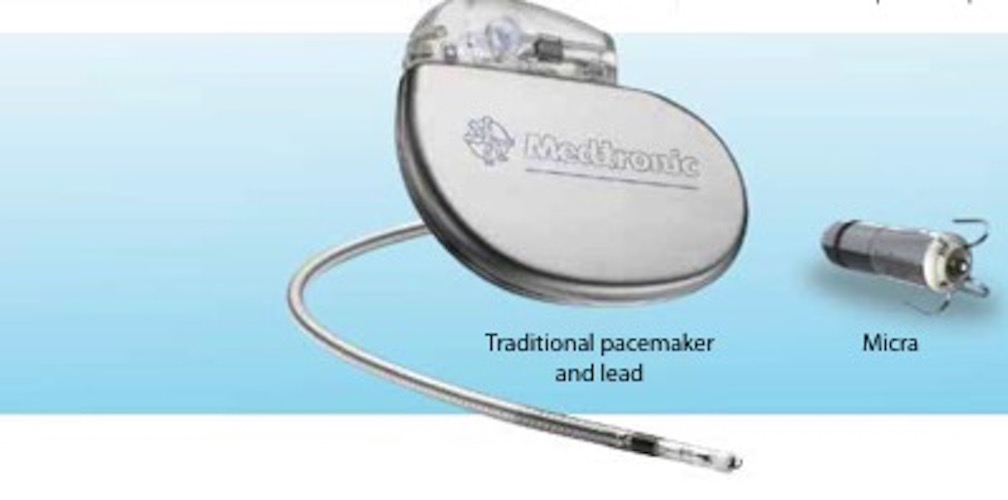
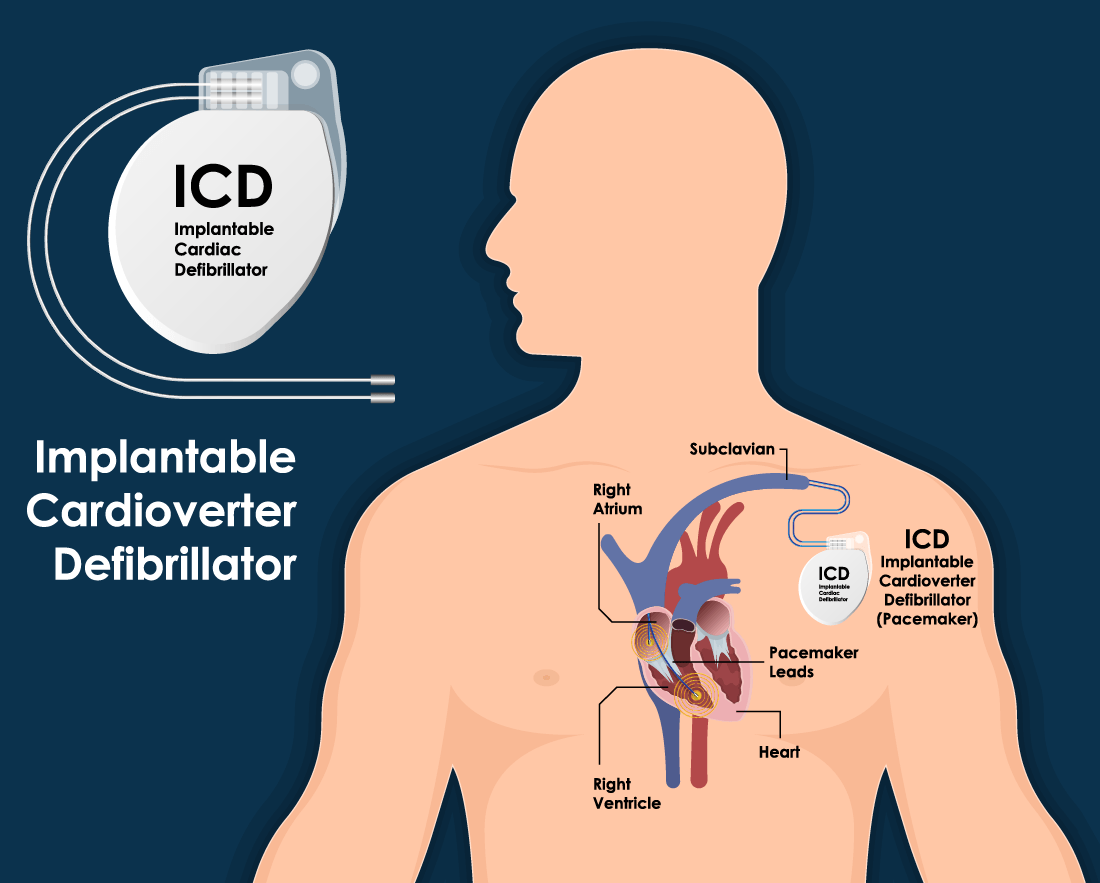
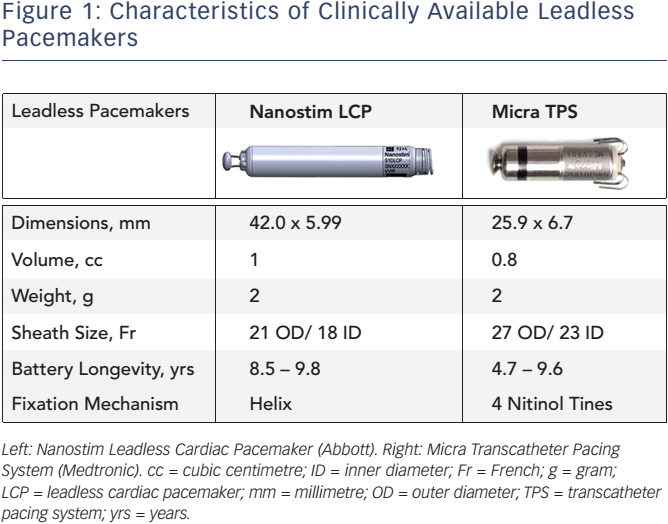
Leadless Pacemaker “Micra” Implantation - Phoenix, AZ & Tempe, AZ: Arizona Heart Arrhythmia Associates
Perioperative Interrogation of Medtronic Cardiovascular Implantable Electronic Devices: A Guide for Anesthesiologists
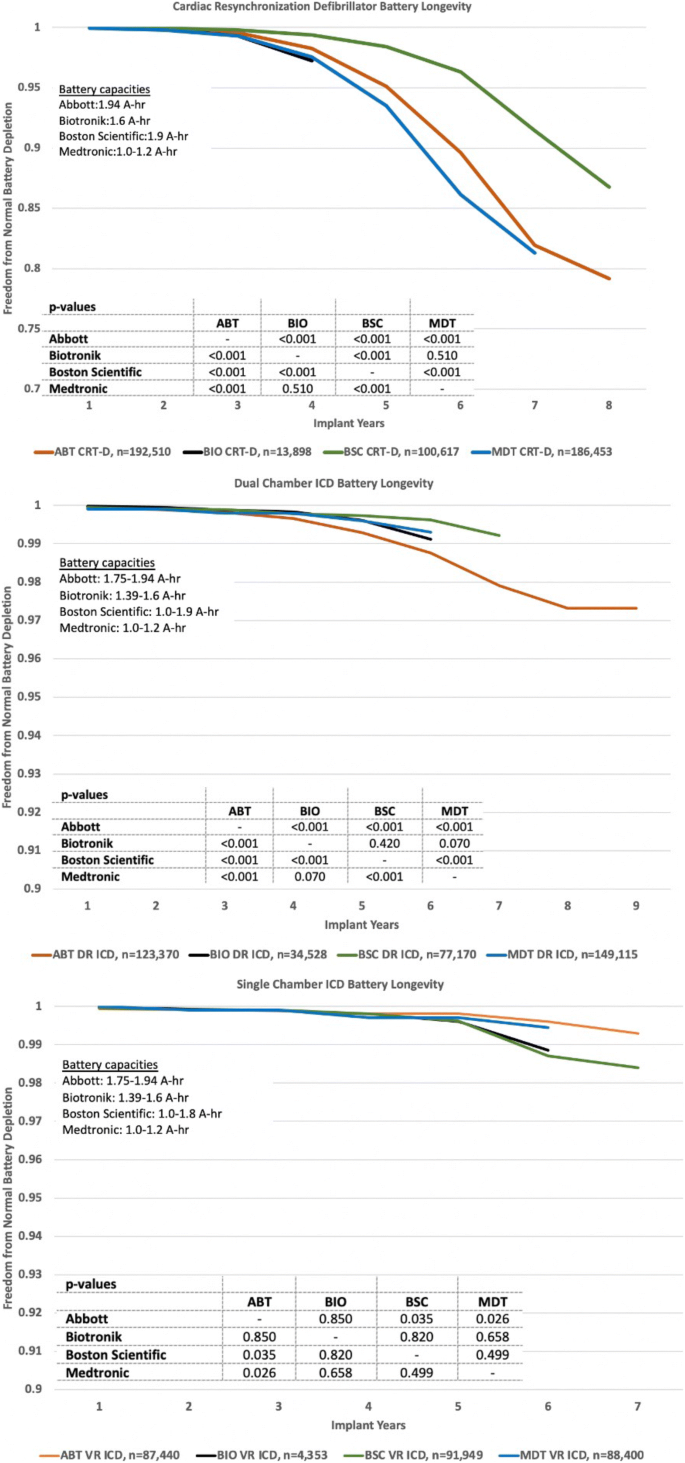
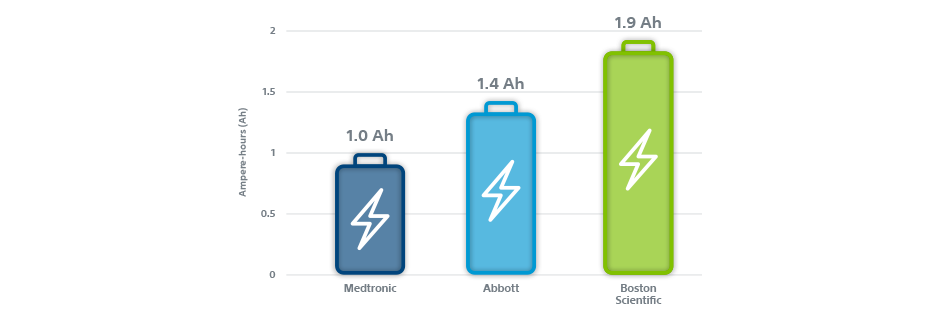
Reliability and longevity of implantable defibrillators | Journal of Interventional Cardiac Electrophysiology

Medtronic Announces CE Mark of First ICD System to Allow for Full-Body MRI | Imaging Technology News

Evolution of extravascular implantable cardioverter-defibrillator therapy for ventricular arrhythmias - Heart Rhythm O2
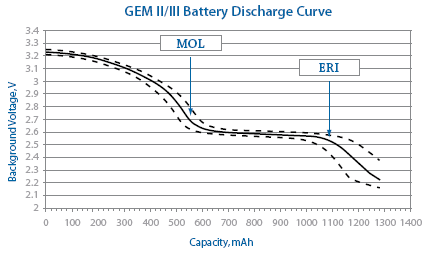
Gem II DR/VR and GEM III DR/VR/AT ICD Battery Discharge Behavior | Medtronic CRHF Product Performance eSource
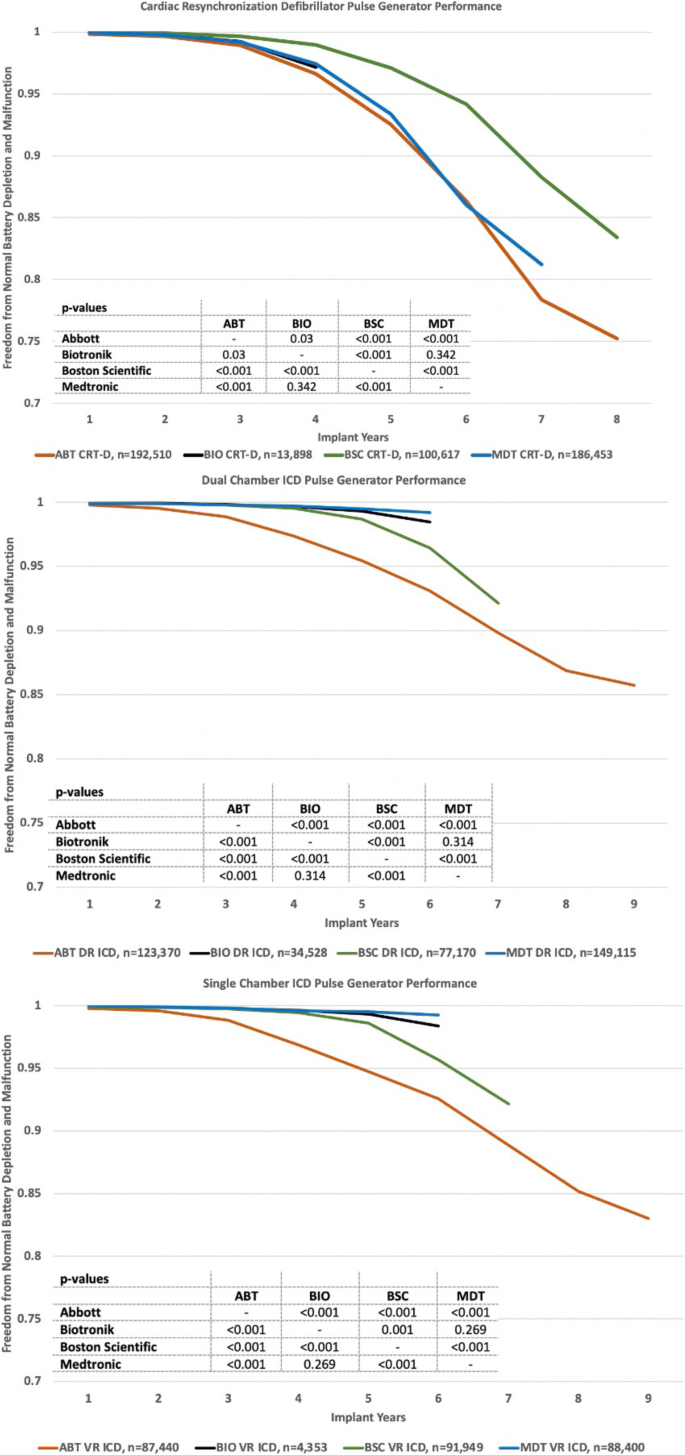

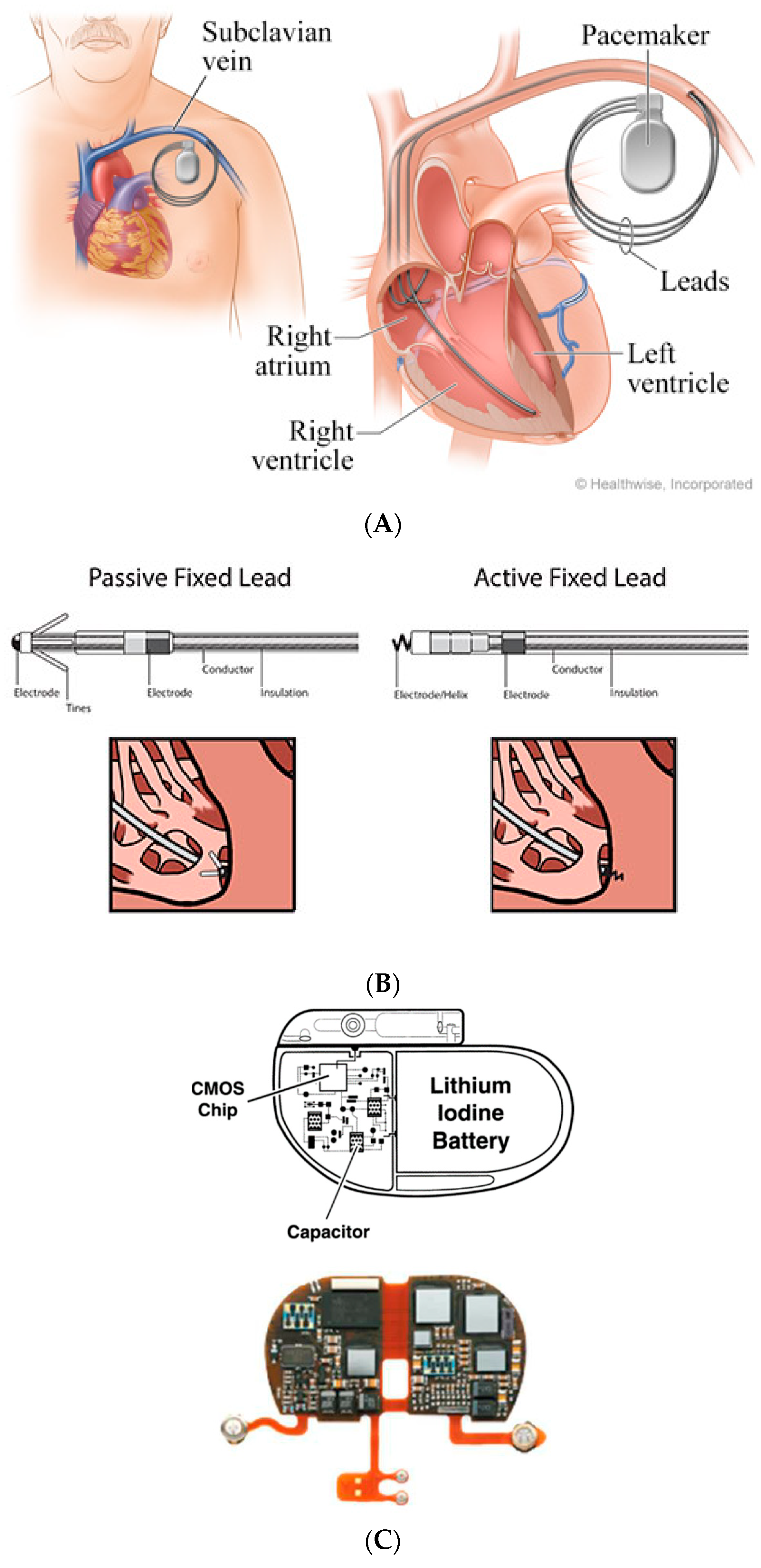



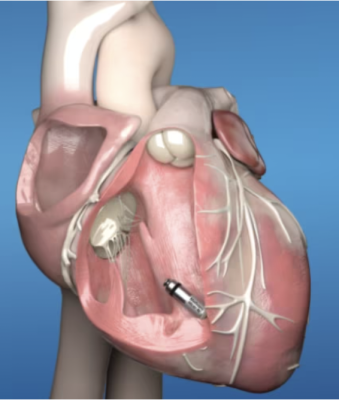
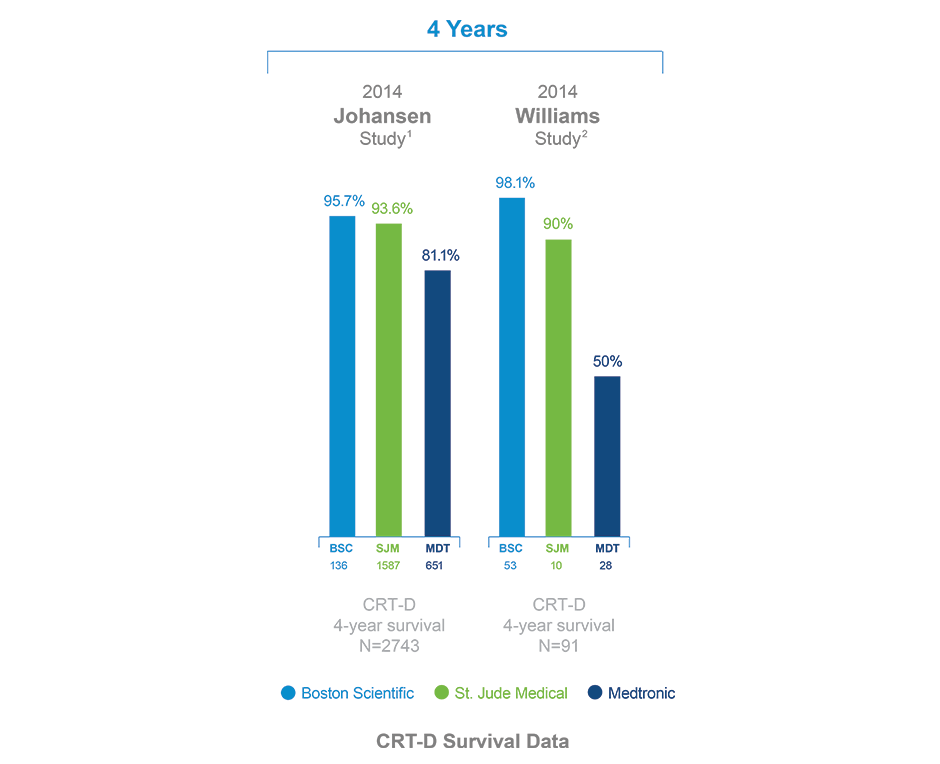



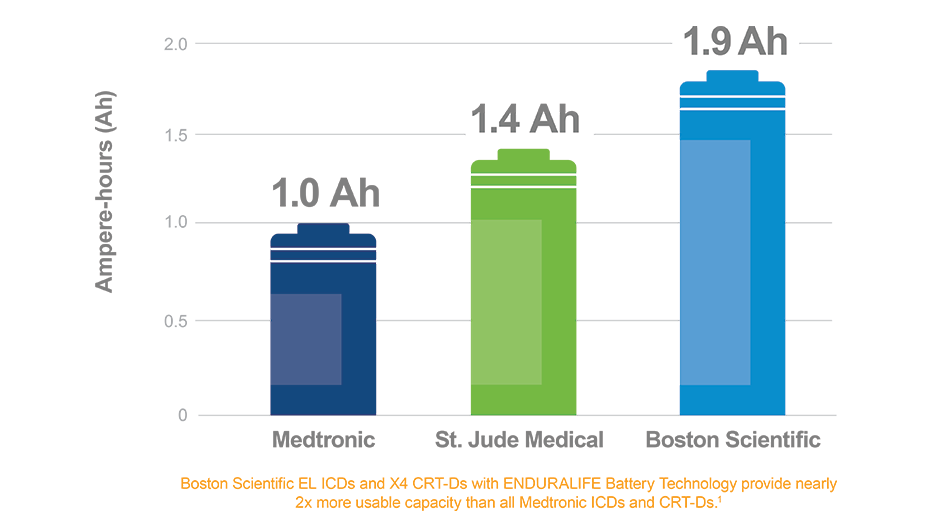


/arc-anglerfish-arc2-prod-dmn.s3.amazonaws.com/public/AT25LTER4G66PEKAEFOT53DQGI.png)
:max_bytes(150000):strip_icc()/135930100-56a470bd5f9b58b7d0d6fc82.jpg)